When you pick up a prescription, you might see two bottles on the counter: one with a familiar brand name, another with plain white labeling and no logo. The price difference is obvious - the generic is cheaper. But is it the same drug? This question comes up often, especially when someone’s health depends on consistent results. The answer isn’t always clear, thanks to confusing labeling and pharmacy practices. But here’s the truth: authorized generics aren’t just similar to brand-name drugs - they’re identical.
What Exactly Is an Authorized Generic?
An authorized generic is the exact same medication as the brand-name version, made in the same factory, using the same ingredients, and following the same production process. The only difference? It doesn’t have the brand name on the bottle. No marketing, no fancy packaging, no logo. Just the drug itself. The U.S. Food and Drug Administration (FDA) defines it plainly: an authorized generic is a brand-name drug sold without the brand name on the label. It’s not a copy. It’s the real thing, just stripped of its branding. These drugs are produced by the original manufacturer under their own New Drug Application (NDA), not through the Abbreviated New Drug Application (ANDA) process that traditional generics use. That’s why you won’t find them listed in the FDA’s Orange Book - they’re not considered generics in the regulatory sense. They’re the brand product, repackaged.Why Do They Exist?
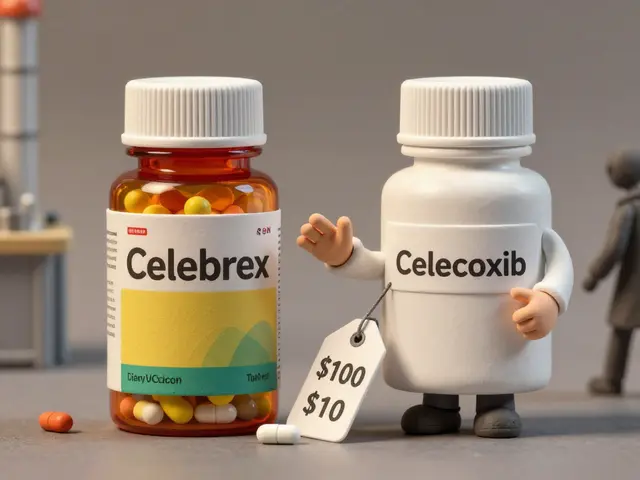
You might wonder why a company would sell its own drug under a different label. The answer is business strategy. When a brand-name drug’s patent expires, other companies can start making cheaper versions. To protect their market share, the original manufacturer sometimes launches its own generic version - an authorized generic - right alongside the competition. This lets them keep some revenue while undercutting the price of traditional generics. It’s a smart move. Authorized generics often cost 15-30% less than the brand-name version but are still 10-20% more expensive than traditional generics. That middle ground gives patients a trusted option without the premium price. And because they’re made by the same company, there’s no risk of formulation changes or supply chain surprises.Are They Really the Same as Brand-Name Drugs?
Yes. Absolutely. Authorized generics contain the same active ingredient, same inactive ingredients (fillers, dyes, binders), same strength, same dosage form, and same manufacturing process as the brand-name drug. That means the way the drug dissolves, how fast it enters your bloodstream, and how long it stays in your system - all of it - is identical. Traditional generics, on the other hand, must prove they’re “bioequivalent” to the brand. That means their absorption rate must fall within 80-125% of the brand’s. It’s a wide range. Two different generic versions of the same drug can have slightly different release profiles. Authorized generics don’t need to prove anything - because they’re not different. They’re the same product. A 2018 study tracking over 5,000 patients switching from brand-name drugs to generics found no meaningful difference in hospital visits, emergency room trips, or medication adherence between those who switched to authorized generics and those who stayed on the brand. In fact, researchers used authorized generics as the gold standard to test whether traditional generics were truly equivalent. The conclusion? Generics performed just as well. And authorized generics? They were indistinguishable from the brand.What About Safety and Quality?
The same FDA inspectors who check the brand-name factory also inspect the facility making the authorized generic. Same equipment. Same quality controls. Same batch records. The FDA requires all drug manufacturers - whether they make brand-name drugs or generics - to follow Current Good Manufacturing Practices (cGMP). There’s no separate standard for authorized generics because they’re not a different product. Even the risk management plans - like REMS programs for high-risk drugs - stay the same. If the brand-name version has special safety requirements, the authorized generic carries them too. No shortcuts. No compromises. Patients with chronic conditions - like asthma, epilepsy, or high blood pressure - often worry about switching. But data from the Asthma and Allergy Foundation of America shows that 87% of users reported no difference in effectiveness when switching from brand-name Singulair to its authorized generic. Only 8% noticed minor changes, and those were likely due to differences in the inhaler mechanism or pill shape, not the drug itself.
How Do They Compare to Traditional Generics?
Here’s where things get messy. Traditional generics are made by different companies, sometimes overseas. They can use different fillers, coatings, or manufacturing methods. While they’re still safe and effective, those small differences can matter for some people - especially those sensitive to dyes, lactose, or certain binders. Authorized generics avoid that entirely. If your body reacts poorly to a traditional generic, it might be because of an inactive ingredient. Switch to the authorized version, and that problem disappears - because the inactive ingredients haven’t changed. Think of it this way: a traditional generic is like a well-made copy of a painting. An authorized generic is the original painting, just hung in a different frame.What Do Pharmacists and Doctors Say?
Most pharmacists know the difference. A 2022 survey by the National Community Pharmacists Association found that 78% of independent pharmacists consider authorized generics interchangeable with brand-name drugs without needing a doctor’s approval. That’s because they’re not just similar - they’re the same. Doctors who understand the system often recommend authorized generics to patients who’ve had bad experiences with traditional generics. Dr. Janet Woodcock, former head of the FDA’s drug center, put it bluntly: “Authorized generics are the brand product without the brand name on the label - they are identical in every way.” But confusion still happens. On Reddit’s r/pharmacy, users report cases where pharmacists mistakenly tell patients the authorized generic is “different” or “not as strong.” That’s not true. It’s the same drug. The confusion comes from lack of training or outdated labeling systems.Are They Covered by Insurance?
Yes. Most insurance plans treat authorized generics the same as traditional generics. That means lower copays - often the same as the cheapest generic option. Some plans even prefer them because they’re more predictable in performance. Medicare Part D, under the Inflation Reduction Act of 2022, has made it easier for seniors to access low-cost medications. Authorized generics fit perfectly into that goal. They’re affordable, reliable, and backed by the same safety data as the brand.
How Common Are They?
As of 2023, there are over 387 authorized generic products on the U.S. market. That’s about 12% of all generic drugs. And the trend is growing. Of the top-selling brand-name drugs, 68% launch an authorized generic within six months of patent expiration. The market for these drugs hit $18.7 billion in 2022 and is growing at nearly 10% a year. Why? Because patients and providers are realizing: you don’t need to pay extra for peace of mind. The brand-name drug is already available - just without the logo.What Should You Do?
If you’re on a brand-name drug and cost is a concern, ask your pharmacist: “Is there an authorized generic for this?” Don’t assume the cheapest option is the best. Sometimes, the authorized generic is only a few dollars more than the traditional generic - but it’s the exact same drug. If you’ve had a bad experience with a traditional generic - maybe you felt off, or your symptoms returned - ask your doctor about switching to the authorized version. It might solve the problem without changing your treatment. And if you’re switching from brand to generic for the first time, ask for the authorized version. You’re not settling for less. You’re getting the same medication, at a better price.Final Thoughts
There’s no mystery here. Authorized generics aren’t a compromise. They’re the original drug, sold honestly. No hidden changes. No hidden risks. Just the same medicine, without the brand name. The FDA, doctors, pharmacists, and millions of patients agree: they’re just as good. Maybe even better - because you know exactly what’s inside.Are authorized generics as effective as brand-name drugs?
Yes. Authorized generics are made by the same company, in the same facility, with the same ingredients and manufacturing process as the brand-name drug. They are identical in every way except for the label. Clinical studies show no difference in effectiveness, safety, or how the body responds.
Why are authorized generics more expensive than regular generics?
Authorized generics usually cost more than traditional generics because they’re made by the original brand manufacturer. They don’t have the same cost-cutting pressures as third-party generic makers. But they’re still 15-30% cheaper than the brand-name version, making them a middle-ground option for patients who want certainty without the premium price.
Can I trust an authorized generic if it looks different from the brand?
Yes. The appearance - color, shape, or markings - can change because the packaging and labeling are different. But the active ingredient, dosage, and how the drug works in your body remain unchanged. The FDA requires that all versions, including authorized generics, meet the same strict standards for dissolution and absorption.
Do authorized generics have the same side effects as brand-name drugs?
Yes. Since the ingredients and formulation are identical, side effects are the same. If you’ve experienced side effects from the brand-name version, you may experience them with the authorized generic too. But if you had issues with a traditional generic due to different fillers or dyes, switching to the authorized version may reduce those problems.
How do I know if my prescription is an authorized generic?
Ask your pharmacist. Authorized generics often have the brand name listed as the manufacturer on the bottle, even if the label is plain. You can also check the FDA’s website or ask your doctor. Some pharmacies label them as “Authorized Generic” or “Same as [Brand Name].” If it’s not clear, don’t assume - ask.





Geraldine Trainer-Cooper
Look i get it everyone wants to save money but this whole authorized generic thing is just corporate theater
Same factory same ingredients sure but why does the brand even bother making it if theyre not trying to control the market
Its not about quality its about profit margins wrapped in a white label
brenda olvera
Im so glad someone finally explained this clearly
I switched to an authorized generic for my blood pressure med last year and honestly i felt better than when i was on the brand
No dizziness no brain fog just steady control
Turns out my body was reacting to the dye in the cheap generic not the medicine itself
Thanks for the post this made me feel less crazy for asking my pharmacist to check
Priya Ranjan
How can you trust a system where the same company makes the brand and the generic
Its not transparency its manipulation
Theyre just exploiting the loophole to keep you paying more than you should
Real generics should be the only option and if the brand is too expensive then let the market force innovation
Stop romanticizing corporate strategy as patient care
pallavi khushwani
I think this is actually really beautiful in a quiet way
Here we have this drug that saves lives and the company decides to offer it without the branding
No marketing no hype just the medicine
It feels like a quiet act of integrity
Like theyre saying hey we dont need to sell you a story to sell you the truth
Its not about profit it about access
And honestly in a world full of noise this is the kind of quiet honesty we need more of
Nigel ntini
There is a critical distinction here that must be emphasized
Authorized generics are not generics in the regulatory sense
They are the original product under a different label
This means they are subject to the same batch controls quality assurance and pharmacovigilance protocols as the branded version
Traditional generics must demonstrate bioequivalence
Authorized generics are bio-identical by definition
This is not semantics it is pharmaceutical science
Kenny Pakade
Ugh stop with the corporate propaganda
Of course theyre the same because they come from the same factory
Thats not genius thats just capitalism
Theyre not doing this for you theyre doing this to crush competitors and keep their profit margins
And dont get me started on how they make you pay 15 more for the same pill
Just give me the real generic and shut up
Brooke Evers
I just want to say how much this post means to me
I have a child with epilepsy and weve been through so many generic switches
One time the seizure frequency spiked and we thought it was the meds
Turns out it was a different filler in the generic
When we found the authorized version everything stabilized
It took us months to figure it out because no one told us this option existed
So thank you for explaining this so clearly
If you or someone you know is struggling with medication changes please ask for the authorized generic
It could make all the difference
You are not being difficult you are being smart
And you deserve to have the best possible treatment without being made to feel like youre asking for too much
Ashish Vazirani
Are you serious
India makes 40% of the world’s generic drugs
And you’re telling me an American company’s version is somehow better
This is cultural imperialism disguised as science
Our generics are cheaper safer and just as effective
Why should we believe your corporate narrative over our own experience
Stop pushing your brand obsession on the rest of the world
Dan Cole
Let me break this down for you
Authorized generics are not a marketing gimmick
They are the only true bioequivalent option
Traditional generics have a 25% window for absorption variability
That means two different pills labeled the same can behave differently in your body
Authorized generics have zero variability because they are identical
The FDA does not require this
They do it because they can
This is not about trust
This is about physics chemistry and pharmacokinetics
If you think a $3 generic is just as good as an $8 authorized generic you are not just wrong
You are putting your life on the line for a few dollars